PGT
(Pre-Implantation Genetic Testing)
PGT
Preimplantation genetic testing (PGT) comprises a wide range of molecular techniques that are utilized during assisted reproduction and in-vitro fertilization (IVF) treatment for a range of indications prior to implantation.
PGT, coupled with expanded carrier screening and increased parental genomic testing, has become an important technology to detect embryos at risk for genetic diseases and chromosomal abnormalities.
To differentiate the specific types of embryo testing, PGT is divided into three subtypes, defined as:
1.PGT-A or Preimplantation genetic testing for Aneuploidies – previously termed as PGS.
2.PGT-M or Preimplantation genetic testing for Monogenic/Single gene disorders – previously termed as PGD.
3.PGT-SR or Preimplantation genetic testing for chromosome Structural Rearrangements.
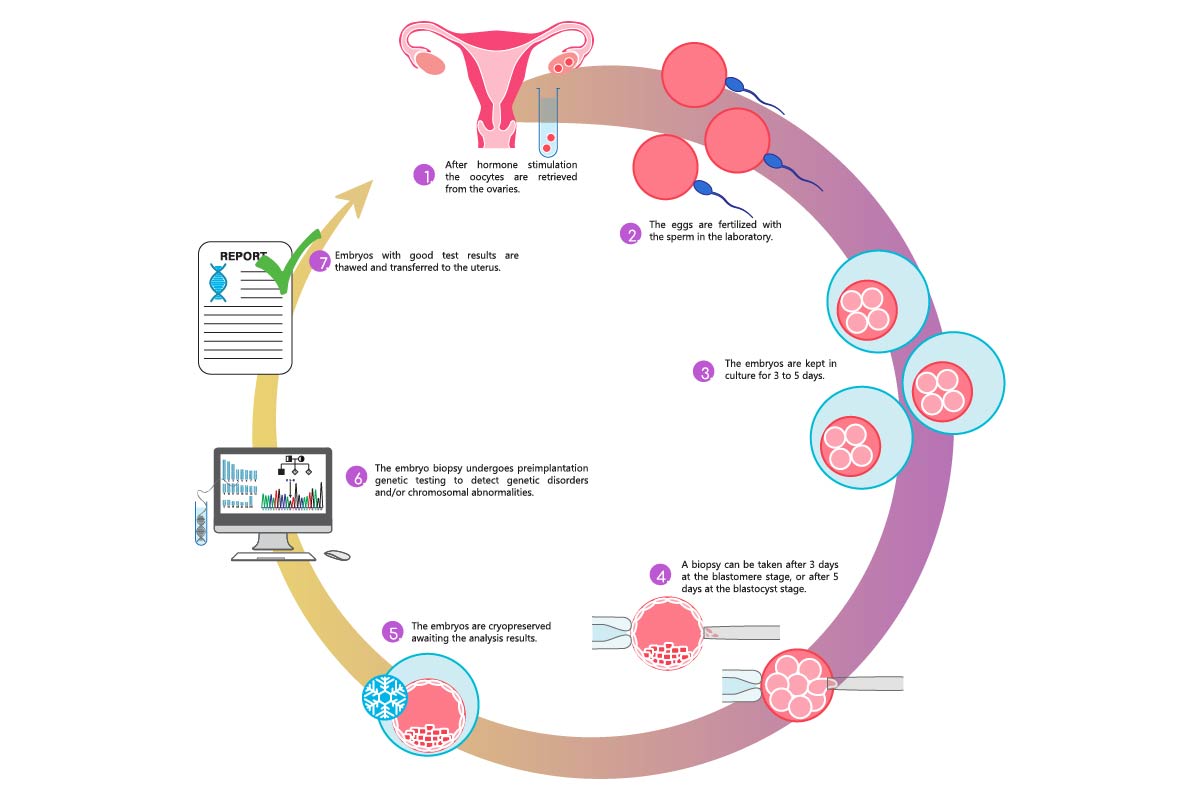
PGT (Pre-genetic testing involves the removal) biopsy and testing of a few cells from the outer layer of the embryos created through the IVF process.
By genetic testing, we can identify the embryos that are healthy and most likely to implant in the uterus, and result in a healthy ongoing pregnancy.
PGT can also provide some insight into the reason that some patients have had difficulty becoming or staying pregnant.
What are the steps of the PGT process?
- Consultation with our Fertility specialist.
- Fertility testing of both partners.
- Genetic testing of both partners (if not already done).
- In-Vitro Fertilization (IVF) – Embryos are created and grown to the Blastocyst stage (day 5 or 6).
- Blastocyst Trophectoderm (outer layer) biopsy of a few cells of each embryo.
- Embryos are frozen/cryopreserved until test results are available.
- Cells removed from the embryos are sent for genetic testing (PGT-A or PGT-M or PGT-SR according to the indication).
- Result usually arrives in 7-14 days.
- Transfer embryos to the uterus of the patient in a subsequent frozen embryo transfer (FET) cycle.
- Normal extra embryos if any remain frozen for future use.
Is the embryo biopsy safe?
In embryos that have developed to day 5 or 6 (blastocyst stage), we can see the difference between the inner cell mass (cells that will make the baby) and the trophectoderm (cells that will make the placenta).
We are able to safely remove cells from the future placenta without disrupting the baby-making cells.
The experience and expertise of the embryologists is very important in minimizing risks to the embryos.
Our embryologist is highly skilled and experienced in this procedure.
We also believe the long-term risks of blastocyst biopsy to the embryo, pregnancy, and baby to be acceptably low as per studies conducted so far.
Does PGT replace prenatal testing?
Not necessarily. While transferring an embryo that has tested “normal” by PGT is expected to significantly reduce the chance for the future baby/child to develop the genetic disorder in question, it does not entirely eliminate the risk.
A Non-Invasive Prenatal Test (NIPT) or Invasive, diagnostic prenatal tests such as chorionic villus sampling (CVS) and amniocentesis are recommended for those who wish to confirm PGT results in the ongoing pregnancy or for those who have an abnormal NT scan result between 11 to 13 weeks of pregnancy.
Types of PGT
PGT-A stands for Pre-Genetic Testing for Aneuploidies.
In PGT-A, an embryo’s Karyotype is tested for the presence of aneuploidies (missing or additional part or an entire chromosome), which is the leading cause for implantation failure and increased miscarriage rate in women specially in those older than 35 years.
The goal of PGT-A is to identify chromosomally-abnormal embryos and exclude them from transfer into the uterus. By transferring only chromosomally-normal ones, the impact of aneuploidies upon implantation failure and miscarriage can be excluded and the outcomes of IVF treatments/chances of a successful pregnancy can be significantly improved.
PGT-A involves removal and testing of cells from embryos created through the IVF process. By determining how many chromosomes are present in these cells, we can identify the embryos that are most likely to implant in the uterus and result in a healthy ongoing pregnancy.
What will I learn about my embryos through PGT-A?
PGT-A counts the number of chromosomes in each embryo’s biopsied cells. Chromosomes are packages of genes, or DNA, within the cells. Humans normally have 46 chromosomes in each cell; 23 come from the sperm cell and 23 from the egg cell. Embryos with the normal number of chromosomes are considered “Euploid”, and those with an abnormal number of chromosomes are considered “Aneuploid”. Aneuploidy is common in embryos, and can cause failed implantation, miscarriage, or the birth of a child with health problems.
What are the steps of the PGT-A process?
- Consultation with our Fertility specialist.
- Fertility testing of both partners.
- Genetic testing of both partners (if not already done).
- In Vitro Fertilization (IVF) – Embryos are created and grown to the Blastocyst stage (day 5 or 6).
- Blastocyst Trophectoderm (outer layer) biopsy of a few cells of each embryo.
- Embryos are frozen/cryopreserved until test results are available.
- Cells removed from the embryos are sent for genetic testing (PGT-A)
- Result usually arrives in 7-14 days.
- Transfer embryos to uterus of patient in a subsequent frozen embryo transfer (FET) cycle.
- Normal extra embryos if any remain frozen for future use.
For who is PGT-A recommended?
PGT-A is indicated for those couples at increased risk of producing embryos with chromosomal abnormalities, and can also be employed where no familial history of a genetic condition is present.
Indications for PGT-A include:
-Repeated implantation failure (RIF), (when patients have undergone 2 or more IVF treatments resulting in no embryo implantation)
-Recurrent pregnancy loss (RPL)-(2 or more consecutive miscarriages after natural or assisted conception)
– Advanced Age of the female partner (>35 years).
-Abnormal Karyotyping report in one or both partners.
Practical considerations and limitations:
-An abnormal PGT-A result may not be fully representative of the chromosomal makeup of an embryo due to mosaicism, and the trophectoderm(cells forming the placenta)may not be representative of the inner cell mass, which becomes the fetus.
-Risk for misdiagnosis with PGT, which is approximately 1%–5% due to possible allele drop-out, mosaicism, or contamination.
-A follow-up diagnostic test (CVS or amniocentesis) can be offered when indicated after discussing all the pros and cons once pregnancy is achieved.
Preimplantation genetic testing for chromosome Structural Rearrangements or PGT-SR is an effective procedure for the detection of embryos having chromosome abnormalities which are hereditary due to one or both parents having a balanced chromosome “structural rearrangement” (such as translocations or inversions).
PGT-SR reduces the risk of having a pregnancy or child with an unbalanced structural abnormality, which involves extra or missing genetic material and typically results in a pregnancy loss.
For which chromosomal conditions can PGT-SR be performed?
PGT-SR is available for most inherited structural chromosome abnormalities that were identified through a Karyotype (chromosome study on blood). However, the ability to offer PGT-SR is dependent on the particular chromosome rearrangement in the parent and on the technologies that are available.
Is PGT-A (preimplantation genetic testing for aneuploidy) recommended along with PGT-SR?
Yes. Due to the high rate of sporadic chromosome abnormalities in embryos (30-80%, depending on maternal age), even from healthy, young, or fertile individuals, PGT-A can provide valuable information and help us find the embryos that are most likely to result in a successful pregnancy. Therefore, we always recommend PGT-A in conjunction with PGT-SR cycles. Both tests can be done simultaneously on the same biopsy, usually using the same technology, and adding PGT-A does not change the overall process or the timeline for results.
What are the steps of the PGT- SR process?
- Consultation with our Fertility specialist.
- Fertility testing of both partners.
- Genetic testing of both partners (if not already done).
- Case review by the PGT lab.
- In-Vitro Fertilization (IVF) – Embryos are created and grown to the blastocyst stage (day 5 or 6).
- Blastocyst Trophectoderm biopsy of 5-10 cells (from the future placental cells of each embryo).
- Embryos are frozen/cryopreserved until test results are available.
- Cells removed from the embryos are sent for genetic testing (PGT-A & PGT-SR)
- Result arrives in 7-14 days.
- Transfer embryos to uterus of patient in a subsequent frozen embryo transfer (FET) cycle.
- Normal extra embryos if any remain frozen for future use.
PGT-M stands for Pregenetic testing for Monogenic/single gene disorders.
PGT-M is used to help individuals or couples reduce their risk to have a child with a known inherited disorder caused by mutations in a single gene(“monogenic”).
PGT-M can be used to identify embryos at risk of having a specific genetic or chromosomal condition.
If you and/or your partner are diagnosed with the risk of passing on a serious genetic condition to your children, preimplantation genetic testing for monogenic / single gene disorders (PGT-M), previously named Preimplantation genetic diagnosis (PGD), can offer a hopeful alternative.
By performing DNA testing on embryos and transferring only those embryos without the inherited condition into the uterus, genetic diseases can be prevented. Hence, IVF treatment is necessary in order to create and test the embryos prior to implantation.
How is PGT-M done?
For this to be performed, patients have to undergo IVF (In vitro fertilization) to create embryos. A few cells from the outer trophectoderm layer of each embryo are then removed with the help of laser assisted embryo biopsy in the lab. These cells are then sent for genetic testing.
The embryos are then frozen/cryopreserved until the results arrive and subsequently transferred in a Frozen embryo transfer cycle (FET).
Only the embryos with normal results are placed into the uterus in the hope of achieving pregnancy and delivering a child without the genetic condition.
For whom is PGT-M recommended?
It is recommended for some couples in order to significantly decrease the chance of having a child with a certain genetic or chromosomal condition.
Examples include:
-A couple who has had a previous child or pregnancy with a serious genetic condition.
-A woman or a man has a serious heritable condition and their children have a 50% risk to inherit the disease gene.
-A couple learn that they are both healthy carriers of the same recessive genetic disease, often without having any family history of the condition.
-A woman or man learn that they are carriers of a chromosome change predisposing them to making eggs or sperm with a chromosomal imbalance that increases the risk for miscarriage or birth defects in their pregnancies.
For which diseases can PGT-M be performed?
Monogenic conditions are those that are caused by mutations in a single gene, rather than by several different genetic and environmental factors.
These conditions may have autosomal dominant, autosomal recessive, X-linked, or mitochondrial inheritance.
PGT-M is available for the vast majority of serious genetic conditions, as long as the particular gene mutation(s) in the family have been identified through DNA testing.
However, the ability to offer PGT-M is dependent not only on the disorder/gene itself but also on the ability to successfully offer a reliable test using available technologies.
Some examples of single-gene conditions for which PGT can be done are:
-Pediatric conditions such as Cystic fibrosis, Spinal muscular atrophy, Thalassemia, and Sickle cell anemia;
-Metabolic disorders such as Tay-Sachs disease and Fanconi anemia;
-Hereditary cancer syndromes such as breast/ovarian cancer predisposition (known to be associated with BRCA1 or BRCA2 genes), Lynch syndrome, and Hereditary diffuse gastric cancer;
-Inherited neurologic and muscular conditions such as Huntington’s disease, Spinocerebellar ataxia, Frontotemporal dementia, Muscular dystrophy, and Myotonic dystrophy.
-X-linked conditions such as Fragile X syndrome, Hemophilia, Adrenoleukodystrophy, and Duchenne muscular dystrophy;
-Inherited heart disease such as Hypertrophic or Dilated cardiomyopathy, Long QT syndrome, and other arrhythmias; and hundreds of other autosomal recessive, autosomal dominant, and X-linked genetic disorders.
Is PGT-A (chromosome screening of the embryos) recommended along with PGT-M?
Yes. Due to the high rate of sporadic chromosome abnormalities in embryos (30-80%, depending on maternal age), even from healthy, young, or fertile individuals, PGT-A can provide valuable information and help us find the embryos that are most likely to result in a successful pregnancy. Therefore, we recommend performing a PGT-A in conjunction with PGT-M cycles. These tests can be done simultaneously on the same biopsy, and adding PGT-A to a PGT-M IVF cycle does not change the overall process or the timeline for results.
What are the steps of the PGT-M process?
- Consultation with our Fertility specialist.
- Fertility testing of both partners.
- Genetic testing of both partners to identify the specific disease mutation in the family (if not already done).
- PGT-M custom test development by the genetic lab.
- In Vitro Fertilization (IVF) – Embryos are created and grown to the blastocyst stage (day 5 or 6).
- Blastocyst Trophectoderm biopsy of 5-10 cells (from the future placental cells of each embryo).
- Embryos are frozen/cryopreserved until test results are available.
- Cells removed from the embryos are sent for genetic testing (PGT-A and PGT-M)
- Result will arrive in 7-14 days.
- Transfer of the embryo(s) reported “normal” into the uterus of the patient in a subsequent frozen embryo transfer (FET) cycle.
- Normal extra embryos if any remain frozen for future use.
How do I get started with PGT?
We recommend making an appointment for consultation with our doctor.